Preventive Corrective Action
Preventive corrective action may require many integral processes that function together for best results. These processes can include audits, change control procedures, customer complaints management, etc. At the core of this is your CAPA management processes, or in other words, Corrective and Preventative Action procedure.
CAPA (Corrective And Preventative Action)
CAPA, according to an online source, "is a concept within Good Manufacturing Practice (GMP),"1 and can be explained (at least partially) in six or eight steps: For eight steps please review this.
8D Manager Software with 8D, 9D, 5Y and 4M report generator. Your corrective action software for managing, measuring, and reporting issues.
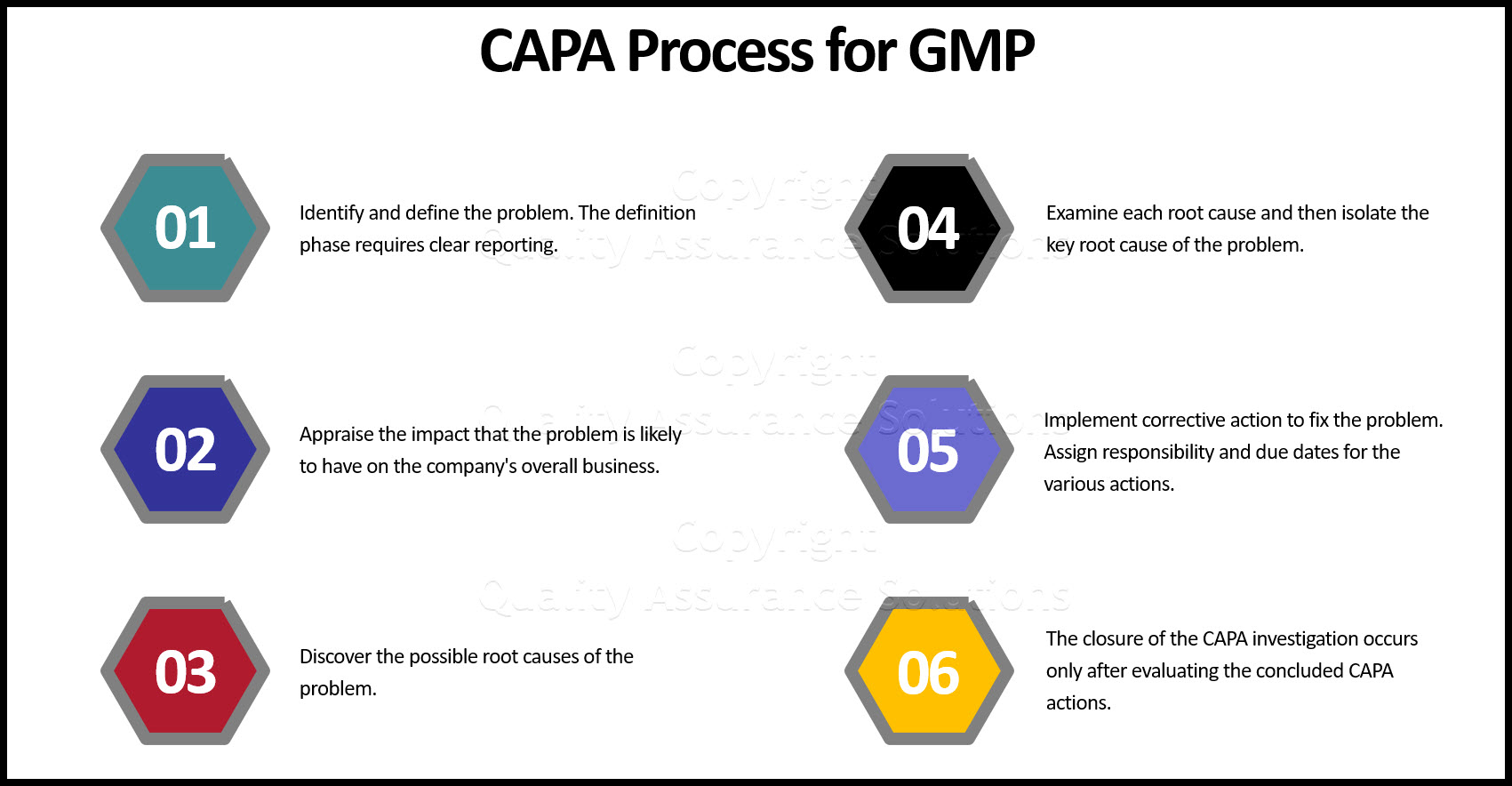
Quality System CAPA Procedure #1: Definition
When a company seeks to implement their CAPA process, they must identify and define the problem. The definition phase requires clear reporting (i.e. documentation). Document how you gathered or deduced the problem information. Provide proof that the problem exists.
Quality System CAPA Procedure #2: The Appraisal
Procedure #2 of the preventive corrective action focuses on appraisal. Here you determine the impact that the problem is likely to have on the company's overall business. Study the possible impacts related to costs, function, product quality, safety, reliability, and/or customer satisfaction issues. Include possible risk to customers, employees and any form of necessary remedial actions.
After pinpointing the impacts and risks then you determine the seriousness of the issue. After years of experience, companies can associate certain risks and/or impacts with varying levels of seriousness. These levels of seriousness act as guides to corrective and preventative actions.
Quality System CAPA Procedure #3: Discovery
If a remedial action has not resolved the issue at hand during phase #2 then undertake the discovery phase of the CAPA process.
Before beginning the discovery investigation determine the goals for the CAPA action. These important goals act as the guidelines for whether or not the CAPA action is resolved by phase #6.
Develop a strategy for the CAPA investigation itself. The strategy should include a set of specific instructions for determining the contributing root causes of the problem. In addition the strategy should also direct a comprehensive review of all circumstances related to the problem.
8D Manager Software with 8D, 9D, 5Y and 4M report generator. Your corrective action software for managing, measuring, and reporting issues.
Quality System CAPA Procedure #4: Examination
The examination stage of the preventive corrective action isolates the root cause of the problem. This "rooting out" takes place after
First determine all possible and probable cause of the problem. Then collect data that supports and/or refutes these possible causes.
Document and organize every aspect of data during this stage. Depending on your company, this data may come from a variety of sources. This inlcudes testing results and/or review of records, processes, service information, design controls, operations, and any other information that may lead to a determination of the fundamental cause of the problem.
Quality System Preventive Corrective Action Procedure #5: Action And Implementation
During the action and implementation stage you start to make progress to fixing the problem. Here you develop a methodology that prevents the problem from reoccuring. Like the other CAPA stages, this plan must be documented. Assign responsibility and due dates for the various actions.
Companies may also want to consider an automated CAPA software solution which tracks and store all CAPA related forms and records and ensures accountability from CAPA participants. A Preventive corrective action software solution can literally save companies days, months and even years worth of valuable time that can be spent on more valuable endeavors.
To close the implementation phase, the CAPA investigator summarizes any actions taken during phase #5. Be sure to inlclude any changes made to the processes and process documentation within the CAPA documentation.
8D Manager Software with 8D, 9D, 5Y and 4M report generator. Your corrective action software for managing, measuring, and reporting issues.
Quality System CAPA Procedure #6: Closure
The closure of the CAPA investigation occurs only after evaluating the concluded CAPA actions. The evaluation, must not only verify the successful completion of the identified tasks, but also assess the appropriateness and effectiveness of the actions taken. Accomplish this by answering a series of questions.
1) Have all of the objectives been met?
2) Have all recommended changes been completed and verified?
3) Has training and appropriate communications been implemented to assure that all relevant employees understand the situation and the made changes
4) Has an investigation demonstrated that that the actions taken have not had any additional adverse effect on the product or service?
If you answered these four questions successfully then document the proof of the actions' success. The actions have been validated.
Article Source: Marci L. Crane
- QAS Home
- 8D Reports
- Preventive Corrective Action
|
Quality Assurance Solutions Robert Broughton (805) 419-3344 USA |
 |
|
Software, Videos, Manuals, On-Line Certifications | ||
|
450+ Editable Slides with support links | ||
|
Corrective Action Software | ||
|
Plan and Track Training | ||
|
AQL Inspection Software |
|
Learn and Train TRIZ | ||
|
Editable Template | ||
|
Templates, Guides, QA Manual, Audit Checklists | ||
|
EMS Manual, Procedures, Forms, Examples, Audits, Videos | ||
|
On-Line Accredited Certifications Six Sigma, Risk Management, SCRUM | ||
|
Software, Videos, Manuals, On-Line Certifications |