Document Revision Control Guidelines
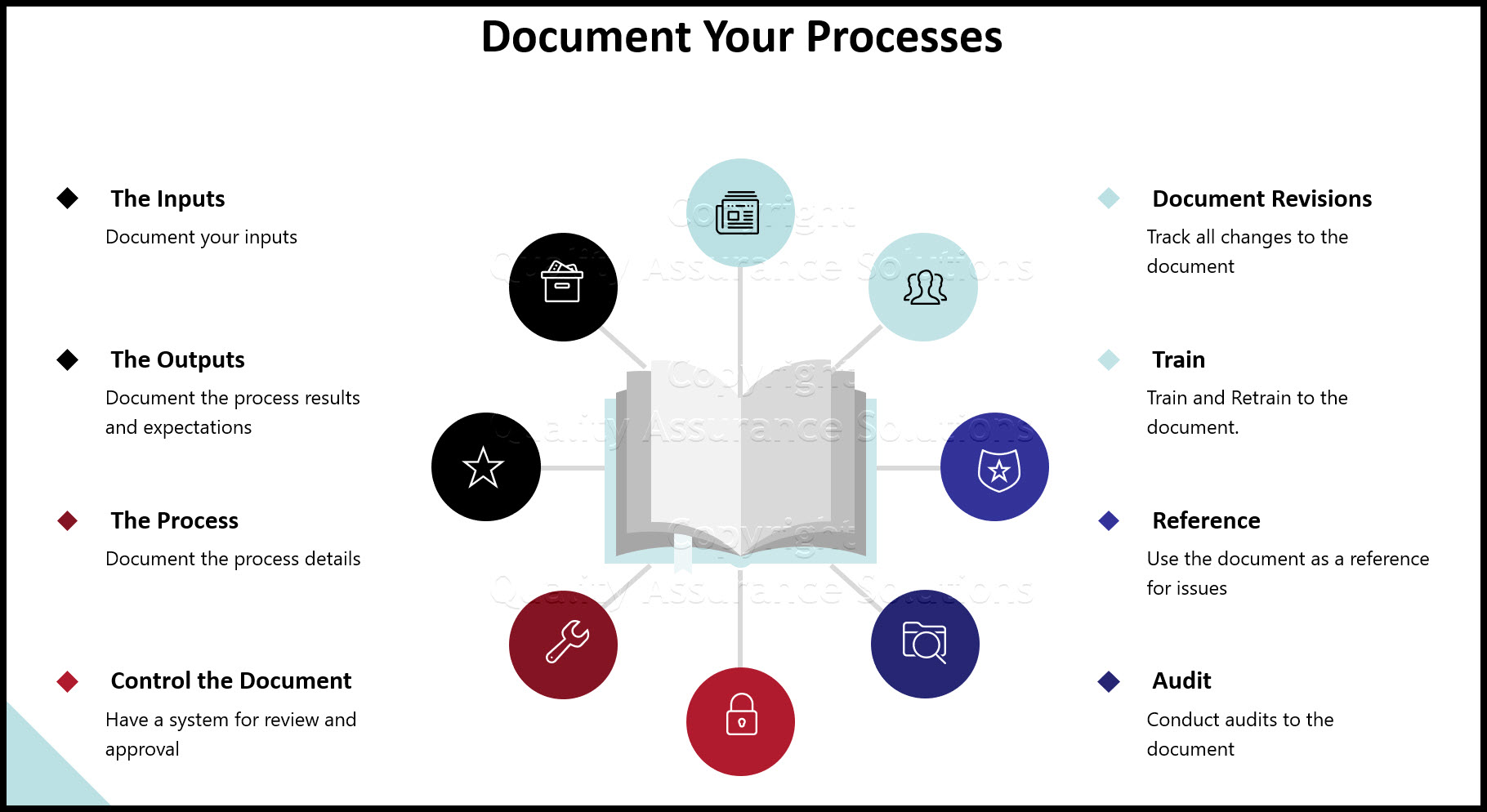
A Document Revision Control system is the spine of your Quality Assurance Program. It is critical for ISO 9001 implementation. This system documents the methods that your organization uses to control, review and approve its documentation.
Your easy to edit ISO 9001:2015 Quality Assurance Manual.
Below is a list of items to consider and document when creating your Document Revision Control System.
- Your numbering scheme
- The process on creating new documentation
- The process on updating the current documentation
- Who is required to review, approve and sign off the documentation
- The difference between master, control and non control copies
- Identification of the Master List
- The method for tracking the location of all documents
- Who is responsible for conducting the document control tasks
- The method for obsoleting unwanted documents
- A mandatory / automatic document review process
- The method used to prevent the unintended use of non control documents or old documents.
- The policy on document readability
- The documentation storage methods
Any information that is used for Product Quality or the Quality Management System must be incorporated into the Document Control system.
This includes:
- Quality Management Systems
- Process Procedures
- Employee Work Instructions
- Specifications
- Software
- Machine Operation Manuals
- CAD Drawings
- Blue Prints
- Process Change Notification
- Quality Record Formats
- Flowcharts
Numbering Scheme
Within your Document Control Revision system, outline your numbering scheme for the documentation. Make the scheme appropriate to your organization. Consider these factors:
- Process step
- Department numbers
- Type of Document
- Document Revision
- Document Locations
- Ease of Identification
- Document Format
- Sequential ID number
- Origin Date
Your ISO 9001:2015 Kit includes Templates, QA Manual, Implementation Guide and a Gap Assessment Internal Audit Tool for ISO 9001:2015
Master Vs. Control Documents
The document control system should have a master document in which there is only one copy of this document. Specify the location of the master document. Ensure that no one can tamper with the master copy.
The master may be digital, hardcopy or a combination of both. Keep the masters in the same location. If the master copy is digital, you could store the files in a directory titled “Master Documentation”. If the master is a hard copy, you could stamp the document with “Master”. The master copy must be readily identifiable as the master.
Maintain a master document list which can be easily accessed as necessary. This list must show the document number, document name, latest revision letter, and origin date of the document.
A control document is normally a copy of the master document. A control copy is usually placed at the point of use. These documents should also be readily recognized as controlled. You want to be sure employees are not using uncontrolled documents.
The location of these controlled document needs to be tracked. When you permanently update the master document, the document control function updates the control documents too.
Includes an easy to edit Calibration Manual, recommended calibration system, reports and templates.
Reviewal / Approval
Document Review / Approval depends on the nature of the document and the organization's management structure. We recommend a mandatory minimum of two people review the document. For small businesses it could be the writer and the primary user of the document. For a process procedure of a larger business it could be the writer, process owner, engineering, quality and production control.
When a document is reviewed and approved, a signature should be used. This could be handwritten or digital. During an audit you must prove that the document was reviewed and approved.
Document Revision Control Function
The document revision control system must specify who is responsible for maintaining the document control system. Document who completes these tasks:
- Designates the master document
- Tracks the locations of control documents
- Distributes the documents
- Assures the documents are reviewed
- Ensures they are legible
- Ensures they are available at all points of use
- Maintains the master list
Your easy to edit ISO 9001:2015 Quality Assurance Manual.
Document Revision Control Changes
When changes occur to a document, the document control system should specify these items:
- How to make a change
- The method that the writer uses to document the changes
- Who is responsible for reviewing and approving the changes
- The process on how the old document is removed from the system and all points of use.
- QAS Home
- Document Control
|
Quality Assurance Solutions Robert Broughton (805) 419-3344 USA |
 |
|
Software, Videos, Manuals, On-Line Certifications | ||
|
450+ Editable Slides with support links | ||
|
Corrective Action Software | ||
|
Plan and Track Training | ||
|
AQL Inspection Software |
|
Learn and Train TRIZ | ||
|
Editable Template | ||
|
Templates, Guides, QA Manual, Audit Checklists | ||
|
EMS Manual, Procedures, Forms, Examples, Audits, Videos | ||
|
On-Line Accredited Certifications Six Sigma, Risk Management, SCRUM | ||
|
Software, Videos, Manuals, On-Line Certifications |